Researchers at Tel Aviv University in Israel have developed a method to detect early signs of Parkinson’s disease at the cellular level using skin biopsies. They say that this capability could enable treatment up to 20 years before the appearance of motor symptoms characteristic of advanced Parkinson’s. Such early treatment could reduce neurotoxic protein aggregates in the brain and help prevent the irreversible loss of dopamine-producing neurons.
Parkinson’s disease is the second most common neurodegenerative disease in the world. The World Health Organization reports that its prevalence has doubled in the past 25 years, with more than 8.5 million people affected in 2019. Diagnosis is currently based on the onset of clinical motor symptoms. By the time of diagnosis, however, up to 80% of dopaminergic neurons in the brain may already be dead.
The new method combines a super-resolution microscopy technique, known as direct stochastic optical reconstruction microscopy (dSTORM), with advanced computational analysis to identify and map the aggregation of alpha-synuclein (αSyn), a synaptic protein that regulates transmission in nerve terminals. When it aggregates in brain neurons, αSyn causes neurotoxicity and impacts the central nervous system. In Parkinson’s disease, αSyn begins to aggregate about 15 years before motor symptoms appear.
Importantly, αSyn aggregates also accumulate in the skin. With this in mind, principal investigator Uri Ashery and colleagues developed a method for quantitative assessment of Parkinson’s pathology using skin biopsies from the upper back. The technique, which enables detailed characterization of nano-sized αSyn aggregates, will hopefully facilitate the development of a new molecular biomarker for Parkinson’s disease.
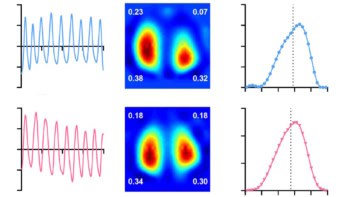
“We hypothesized that these αSyn aggregates are essential for understanding αSyn pathology in Parkinson’s disease,” the researchers write. “We created a novel platform that revealed a unique fingerprint of αSyn aggregates. The analysis detected a larger number of clusters, clusters with larger radii, and sparser clusters containing a smaller number of localizations in Parkinson’s disease patients relative to what was seen with healthy control subjects.”
The researchers used dSTORM to analyse skin biopsies from seven patients with Parkinson’s disease and seven healthy controls, characterizing nanoscale αSyn based on quantitative parameters such as aggregate size, shape, distribution, density and composition.
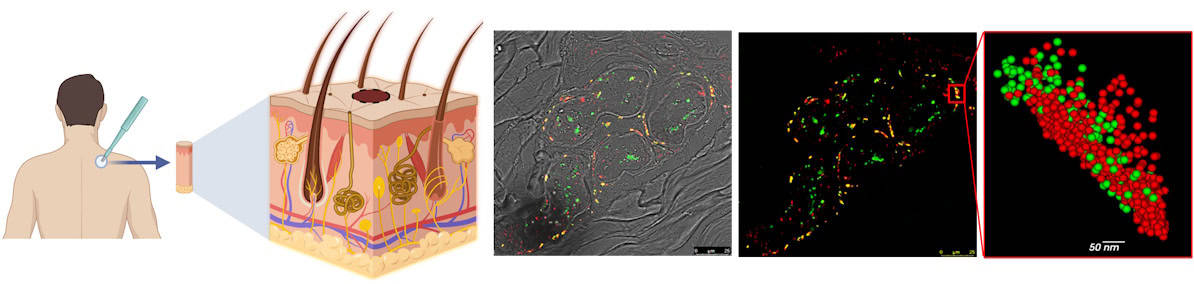
Their analysis revealed a significant decrease in the ratio of neuronal marker molecules to phosphorylated αSyn molecules (the pathological form of αSyn) in biopsies from Parkinson’s disease patients, suggesting the existence of damaged nerve cells in fibres enriched with phosphorylated αSyn.
The researchers determined that phosphorylated αSyn is organized into dense aggregates of approximately 75 nm in size. They also found that that patients with Parkinson’s disease had a higher number of αSyn aggregates than the healthy controls, with larger αSyn clusters (75 nm compared with 69 nm).
“Parkinson’s disease diagnosis based on quantitative parameters represents an unmet need that offers a route to revolutionize the way Parkinson’s disease and potentially other neurodegenerative diseases are diagnosed and treated,” Ashery and colleagues conclude.
In the next phase of this work, supported by the Michael J. Fox Foundation for Parkinson’s Research, the researchers will increase the number of subjects to 90 to identify differences between patients with Parkinson disease and healthy subjects.
Cardiac PET scans could predict onset of neurodegenerative disease in at-risk individuals
“We intend to pinpoint the exact juncture at which a normal quantity of proteins turns into a pathological aggregate,” says lead author Ofir Sade in a press statement. “In addition, we will collaborate with computer science researchers to develop a machine learning algorithm that will identify correlations between results of motor and cognitive tests and our findings under the microscope. Using this algorithm, we will be able to predict future development and severity of various pathologies.”
“The machine learning algorithm is intended to spot young individuals at risk for Parkinson’s,” Ashery adds. “Our main target population are relatives of Parkinson’s patients who carry mutations that increase the risk for the disease.”
The researchers report their findings in Frontiers in Molecular Neuroscience.