The animal kingdom has benefited from millions of years of biological evolution to adapt processes and characteristics to meet specific needs. Using an approach known as bioinspiration, scientists and engineers are employing insights from biology to solve today’s technology challenges and optimize the design of new materials, devices and structures.
Within the medical field, for example, researchers have designed a surgical imaging system based on the amazing eyes of the mantis shrimp, created a space blanket that allows users to control their temperature by mimicking the adaptive properties of squid skin, and fabricated an intraocular pressure sensor based on nanostructures with optical properties first discovered in the wings of a butterfly.
And this week saw the publication of two new research studies exploiting insights from biology for the benefit of human health.
Powered by pangolin scales
First up, the pangolin – the only mammal that’s completely covered in hard scales. These scales connect to the underlying skin, rather than to each other, and overlap in the style of a pine cone, enabling the pangolin to curl into a ball when threatened. And it is these scales that provided the inspiration for Metin Sitti from the Max Planck Institute for Intelligent Systems and his collaborators to design a miniature soft medical robot.

Untethered magnetic soft robots offer the potential to perform minimally invasive medical procedures inside the body. One day, such robots could be guided by magnetic fields to hard-to-reach regions where they can then deliver drugs or create heat. Localized heating can be used to stop bleeding, cut tissue or even ablate tumours. Remote generation of heat, however, requires the use of rigid metallic materials, which can compromise the compliance and safety of soft robots.
“To address this inherent trade-off between effective remote heating at long distances and compliance, we observed how pangolins in nature could still achieve flexible and unencumbered motion despite having keratin scales which are orders of magnitudes harder and stiffer than the underlying tissue layers, simply by organising the keratin scales into an overlapping structure,” write the researchers, in Nature Communications.
With this in mind, Sitti and colleagues designed and built a 20 x 10 x 0.2 mm robot comprising a soft polymer layer and a pangolin-inspired layer of overlapping metal elements. By exposing the robot to a low-frequency magnetic field, the researchers could make it roll up and move about. When exposed to a high-frequency magnetic field, the robot delivered on-demand heating (by over 70°C) at large distances (more than 5 cm) within less than 30 s.
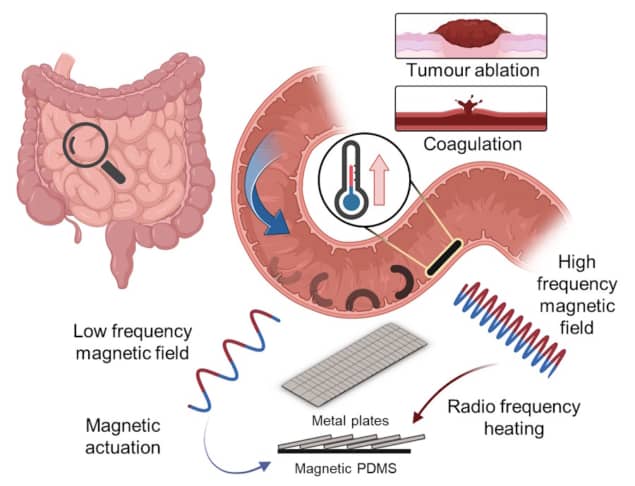
In proof-of-concept experiments on tissue phantoms, the team showed that a 65 mT rotating magnetic field could actuate and move an untethered robot, and that the heating scales could selectively release cargo secured to the robot with beeswax.
To further assess the robot’s clinical potential, the researchers simulated bleeding inside an ex vivo pig stomach and demonstrated that the robot could navigate to the bleeding site and use heat to stop the bleed. They also placed tumour spheroids in direct contact with the heating scales, which destroyed the spheroids after just 5 min of heat at 60 °C.
“Many questions and technical challenges still remain, although surmountable, they require more time and effort. These include the clinical utility and practicality of deploying these robots in clinical scenarios, biocompatibility issues, control and tracking,” says first author Ren Hao Soon. “In my next project, I want to continue pushing these untethered robots closer to the bedside. I hope to work closely with clinicians to identify a real medical need for which such robots might be useful.”
Emulating the octopus bite
The blue-ringed octopus is tiny, vibrant in colour, and one of the world’s most venomous marine animals. Its bite punctures the shell of its prey and then releases tetrodotoxin, a paralysing neurotoxin. “The predatory behaviour of the blue-ringed octopus inspired us with a strategy to improve topical medication,” writes a research team headed up at Sichuan University and Zhejiang University in China.
Intratissue topical medication – a method in which drugs are delivered into tissue surfaces via microneedles – offers rapid action, high drug bioavailability and minimal invasiveness. The approach can be used to inhibit tumour growth, for example, or accelerate healing. Challenges remain, however, such as adhering drug carriers to soft tissue surfaces wetted by bodily fluids and controlling the concentration of drug release.
To overcome these obstacles, first author Zhou Zhu and colleagues created a microneedle patch that provides robust tissue surface adhesion and active-injection drug delivery. Writing in Science Advances, they note that the drug-releasing microneedles work in a manner “inspired by the teeth and venom secretion of the blue-ringed octopus”.
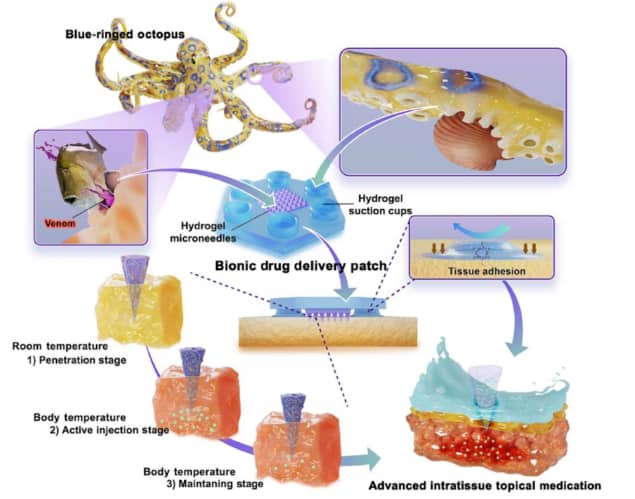
The researchers formed the microneedle patch from a mixture of silk fibroin and the hydrogel pluronic F127 (silk-Fp), adding heat-sensitive PNIPAm hydrogel to enable controlled drug release. The resulting hydrogel microneedles were strong enough to penetrate soft tissue or the mucus barrier.
One challenge, particularly in a humid environment, is to keep the patch stable on the tissue surface. Imitating the design of the suction cups on the octopus’ tentacles, the team created a base layer containing hydrogel suction cups and integrated the microneedles into its centre. The suction cups adhere to tissue via both negative pressure fixation and chemical bonding with tissue proteins. Even after long periods under water, the silk-Fp patch stayed firmly in place on the tissue surface.
To test the functionality of the patch, the researchers loaded the silk-Fp microneedles with the anti-inflammatory drug dexamethasone sodium phosphate (DEX) or the anticancer drug 5-fluorouracil (5-FU), and applied the patches to treat oral ulcers or early superficial tumours in animals, respectively.

Penguin physics
The shape and strength of the microneedles enabled them to puncture into the ulcer or tumour. After entering the target tissue, the microneedles sense the body temperature and provide rapid-onset drug delivery within two hours (as the needles shrink and the PNIPAm transforms from a hydrophilic to a hydrophobic state upon heating). Over the next two days, the microneedles gradually deliver the remaining drug to maintain the therapeutic effect.
The researchers found that the silk-Fp microneedle patch could increase the healing speed of ulcers through DEX release or almost completely halt tumour growth when loaded with 5-FU. “Imitating the blue-ringed octopus biting through the shell of its prey and injecting toxic saliva, the developed silk-Fp MN could actively inject drugs into tissues,” they write.