A US-based research team has developed a new “sonogenetic” technique to activate and control mammalian cells with sound – potentially paving the way for innovative non-invasive versions of deep brain stimulators, pacemakers and insulin pumps. So, what exactly is sonogenetics? What are its potential clinical advantages? And what are the next steps for the research team?
Ultrasound stimulation
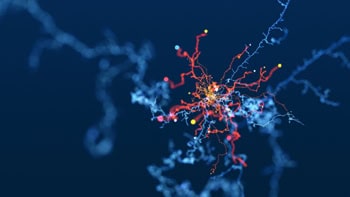
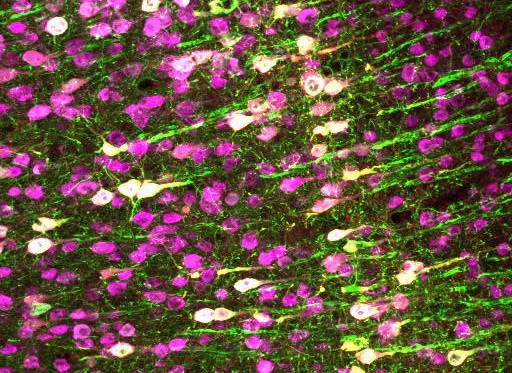
The novel approach combines ultrasound with gene therapy to deliver genes to specific cells in order to manipulate them with sound for therapeutic effect. The results of the research, reported in Nature Communications, show how the team used the method to activate human cells in a dish and to control neurons in the brain and spinal cord of mice.

“Sonogenetics uses ultrasound to control specific cells,” explains senior author Sreekanth Chalasani from The Salk Institute for Biological Studies. “Ultrasound is sound, which is mechanical in nature, and so it causes a small amount of mechanical deflection in the focal zone. We identified a channel protein that can sense this mechanical deflection. So, we can express this channel on a cell that we want to control.”
To test the technique, the researchers delivered the gene for a channel protein (TRPA1) to target cells. After expressing this channel, the cells became sensitive to ultrasound. In studies on mice, the team attached a transducer to the skin of the animals and could then control the target cells using ultrasound stimulation. “This channel is not found on the cell normally, so we are introducing a new functionality by adding this protein to the cell,” Chalasani adds.
Potential clinical applications
According to Chalasani, sonogenetics has a number of key advantages over more invasive existing therapeutic methods. To begin with, in basic terms, the new technique demonstrates how motor neurons in the cortex can move limbs – which was an expected result. He also points to a wide range of potential non-invasive clinical applications, including the replacement of deep brain stimulators, cardiac pacemakers and insulin pumps.
“Cardiac pacemakers are likely to be the first clinical application since there is a gene delivery system to deliver genes to the heart muscle in a human. We don’t have a way to deliver genes to targets in the human brain,” he says.
Current approaches for each of these methods involve surgery and include implanting a device in the body, which comes with an increased risk for infection and/or inflammation. “Our method doesn’t need an implant as the transducer is outside the body. All we have done is deliver a protein to the target cell,” Chalasani explains.

Wireless implant uses optogenetics to control spinal cord activity in mice
Chalasani says that he and his team are already engaged in taking the findings forward – and reveals that they are hoping to identify additional proteins that have increased sensitivity to ultrasound, that can respond to ultrasound at specific frequencies and that can inhibit neurons, not just excite them as they are currently doing.
“Also, we want to continue innovating with the ultrasound delivery devices so that we can deliver appropriate ultrasound stimuli to targets in the brain or body,” he tells Physics World. “We are currently working with mice, but want to extend this to large animals like pigs or primates before translating it to humans.”