
The introduction of photon-counting detectors into CT scanners paved the way for the rise of spectral CT in clinical settings. Such systems employ two or more X-ray energies to create material-specific 3D maps. But since spectral CT is based on X-ray attenuation, it exhibits low contrast when imaging weakly absorbing materials such as biological tissues. As such, high-Z contrast agents are often employed to highlight structures of interest.
In parallel, X-ray phase-contrast imaging is becoming more widely available and gaining attention for both pre-clinical and clinical applications. Phase-contrast techniques, many of which can produce both attenuation and phase-shift maps, offer higher visibility of low-Z materials such as soft tissues.
“Spectral CT has proven effective in a range of applications, from material quantification to image-artefact reduction, while phase-contrast imaging boasts superior visualization of soft and microstructured tissues,” says Luca Brombal from the University of Trieste and INFN. “Building on these bases, we sought to leverage the combined strengths of both techniques.”
Brombal and colleagues, also from University College London, demonstrated the first integration of spectral and phase-contrast CT using a tomographic edge-illumination setup. The project, described in Physics in Medicine & Biology, involved developing an imaging setup that can acquire data with both spectral and phase-contrast properties, alongside the implementation of a material decomposition model.
“The benefits of the combined spectral phase-contrast approach are the possibility to simultaneously produce three mass density maps of specific elements or compounds in the sample, while improving the signal-to-noise ratio, especially of the soft-tissue component, due to phase sensitivity,” Brombal explains.
Material decomposition
The team used an edge-illumination phase-contrast set-up, in which masks placed either side of the sample shape the incident X-ray beam and selectively block the detector. A reference illumination curve is created with no sample in place. Once the sample is inserted, this curve is attenuated and laterally displaced, changes that are then used to retrieve attenuation images and calculate the sample-induced phase shift.
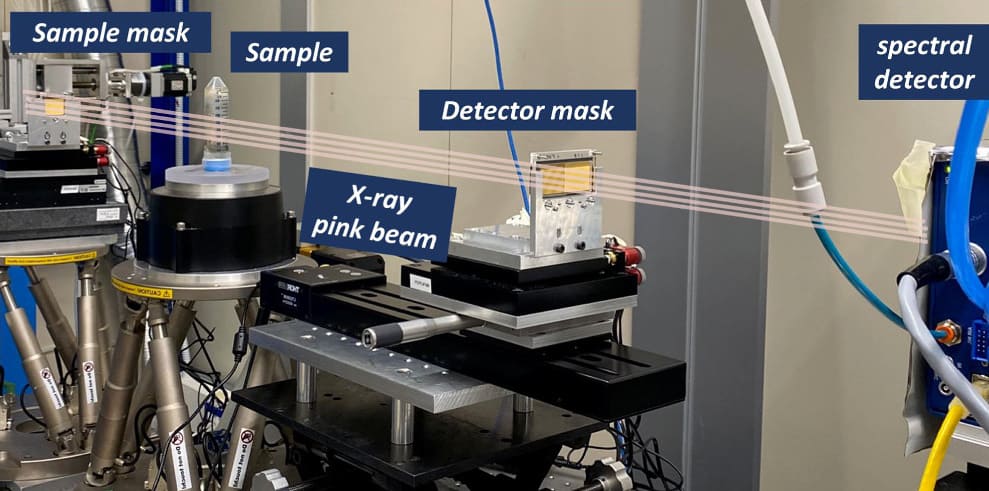
For this study, the researchers employed synchrotron radiation from the Italian synchrotron facility Elettra. They note, however, that translation to a laboratory setup using conventional X-ray tubes should be straightforward. They first scanned a test phantom comprising plastic cuvettes filled with five liquids: calcium chloride solution (370 and 180 mg/ml); iodine solution (50 and 10 mg/ml, similar to concentrations used in iodine-based contrasts); and distilled water.
The imaging system is based on a photon-counting detector with a small-pixel (62 µm) cadmium telluride sensor, operated in two-colour mode to record incoming photons in low- and high-energy bins. The researchers acquired tomographic images of the phantom, recording 360 projections over 180°, with an exposure time of 1.2 s per step and a total acquisition time of 2.9 h.
After reconstructing 3D volumes from the attenuation and phase projections, the team performed material decomposition using three algorithms: spectral decomposition, using the low- and high-energy attenuation reconstructions as inputs; attenuation/phase decomposition, applied to phase and attenuation reconstructions obtained by summing the energy bins; and spectral/phase decomposition, which uses low-energy, high-energy and phase reconstructions.
The spectral/phase decomposition algorithm exhibited the best performance of the three, correctly identifying all materials with no signal contamination across channels and significantly less noise than standard spectral decomposition, due to the low noise of the input phase channel. This algorithm computed values closest to the nominal mass density, with RMS errors of 1.1%, 1.9% and 3.5% for water, iodine and calcium chloride solutions, respectively.
Spectral/phase decomposition also improved the signal-to-noise ratio of the images, by a factor of nine in the water channel and a factor of 1.3 in iodine images, compared with spectral decomposition. In addition, only the spectral/phase decomposition enabled simultaneous quantification of all three material densities.
Biological demonstration
To validate the technique using a biological sample, the researchers imaged ex vivo a laboratory mouse perfused post-mortem with an iodine-based vascular contrast agent. They acquired 720 projections over 360°, with a total exposure time of 5.8 h and a resulting radiation dose of around 2 Gy. They note that for future in vivo applications the delivered dose could be reduced to hundreds of milligray, by optimizing the mask design, for example, or using more dose-efficient acquisition schemes.
To preserve high-resolution details, the researchers reconstructed attenuation and phase images with a 20 µm3 voxel size. Spectral attenuation images showed signal from bones (calcium map) and vasculature (iodine map), but no soft-tissue signal. The phase input reconstruction, meanwhile, revealed soft-tissue structures such as cutaneous and subcutaneous layers and internal organs
Material decomposition using the spectral/phase algorithm clearly separated the vasculature and bones, with no contamination signal, while the phase channel provided good visibility of the formalin-fixed soft-tissue component.
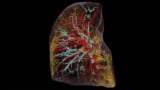
Drawing up a ‘Google Earth’ of the human body
The high resolution of the iodine and calcium images demonstrated that the system can capture blood vessels smaller than 50 µm, as well as the fine trabecular structure of the bone. The researchers also created a 3D rendering of the mouse sample reconstruction after spectral/phase decomposition, which simultaneously visualizes soft tissues, bones and vasculature.
The next step, Brombal tells Physics World, will be to translate this technique from a proof-of-principle study to more compelling scientific cases. “We recently started a new project focused on the application of spectral phase-contrast to osteoarticular research, especially in the context of detection of diseases such as osteoarthritis, and to (quantitative) virtual histology, potentially providing complementary insights alongside conventional pathological analysis of surgical tissue specimens.”



